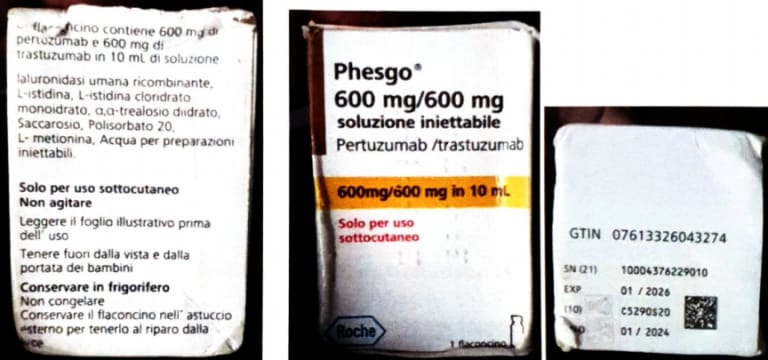
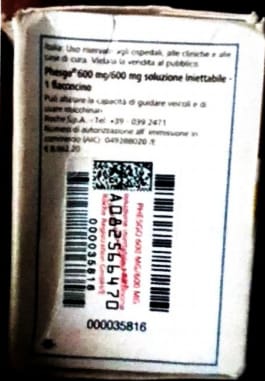
Lagos, Nigeria – The National Agency for Food and Drugs Administration and Control (NAFDAC) has issued a public alert regarding counterfeit Phesgo® 600mg/600mg/10ml injection labelled with batch number C5290S20, falsely stated to be manufactured by Roche S.P.A.
The warning follows a complaint from a Lagos University Teaching Hospital (LUTH-NSIA) doctor about the suspected counterfeit product presented by a patient.
Roche, the Marketing Authorisation Holder (MAH), reviewed the product’s images and confirmed it as counterfeit due to discrepancies, including:
- Non-existent batch number in Roche’s database.
- Language inconsistencies.
- Incorrect tamper evidence labels and missing safety features.
No physical sample was available for chemical analysis.
Product Risks
Counterfeit medicines, like the falsified Phesgo®, pose severe health risks, compromising safety, quality, and effectiveness.
Counterfeit Product Details:
- Product Name: Phesgo® 600mg/600mg/10ml Injection
- Manufacturer (Claimed): Roche S.P.A
- Correct Manufacturer: F. Hoffmann-La Roche Ltd., Switzerland
- Batch No.: C5290S20
- Manufacturing Date: 01/2024
- Expiry Date: 01/2026
Phesgo® is primarily used in breast cancer treatment.
NAFDAC has directed surveillance and mop-up operations to eliminate the counterfeit product from circulation.
Advisory to Healthcare Providers and Consumers
- Obtain medicines from authorized suppliers.
- Verify product authenticity and physical condition.
- Report suspected counterfeit products to NAFDAC via 0800-162-3322, email at sf.alert@nafdac.gov.ng, or the Med-Safety app.
This alert will also be submitted to the WHO Global Surveillance and Monitoring System (GSMS).